Don't Miss Your Opportunity to Share Your Voice at Nexus 2024!
Here's What You Missed at Nexus 2023!
Networking and education were event highlights in Orlando!
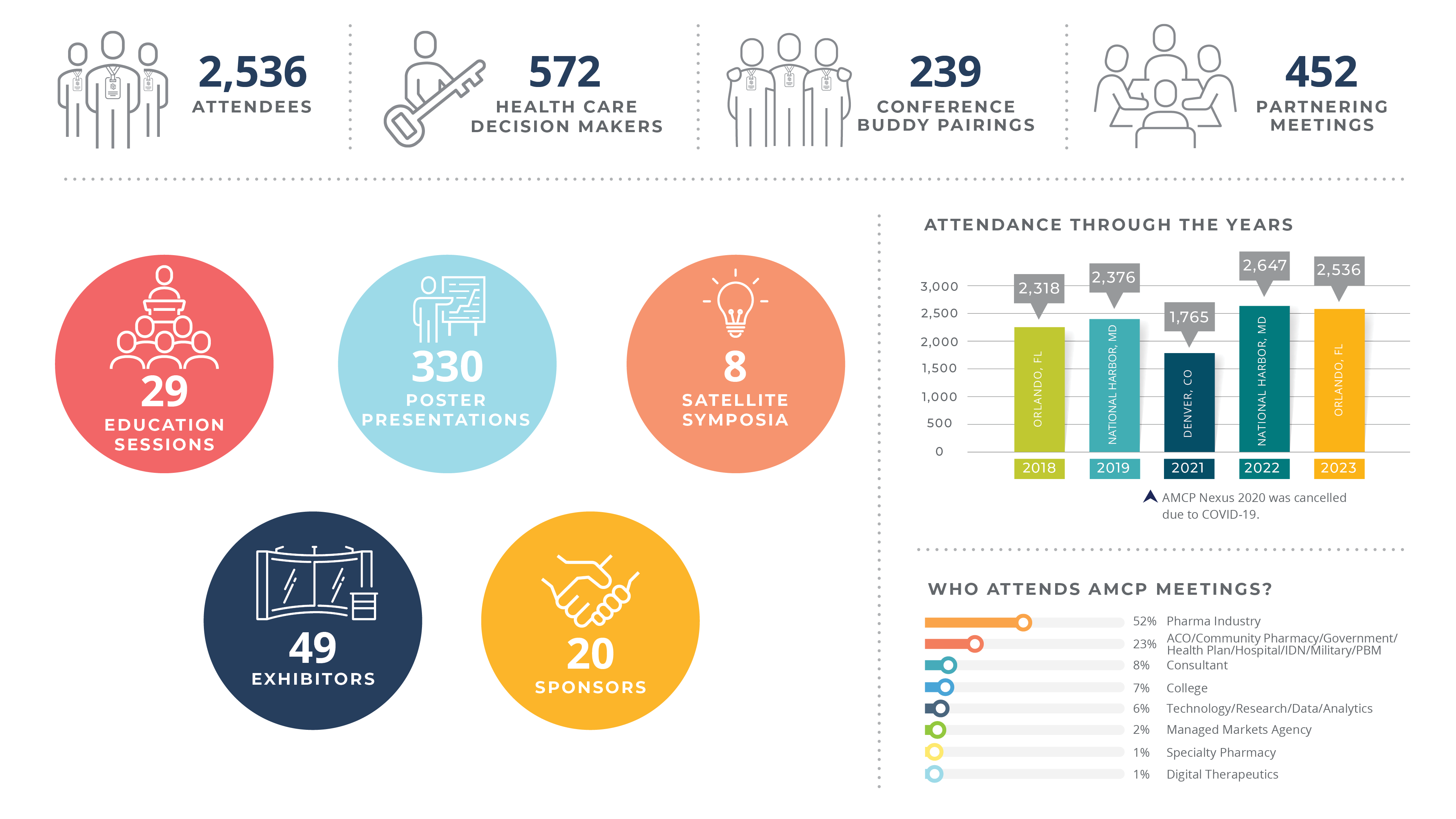
Nexus 2023 attracted nearly 3,000 member and non-member managed care decision-makers to the Orlando Marriott World Center in beautiful Orlando, FL, from Oct. 16–19. Our wrap-up video highlights the fun and excitement our attendees had while networking and building relationships that will drive business in 2024, and here is a write-up of the complex issues discussed at the meeting. Don't miss out this year - be sure to join us in Las Vegas for AMCP Nexus 2024!
Nexus 2023 by the Numbers
Stay Informed
Want to receive the latest information on AMCP Nexus 2024 in Las Vegas, NV from Oct. 14-17? Fill out the form below so you don't miss out on one of managed care pharmacy's most exciting events for 2024!

